4 January 2021
Earlier this year, I posted preliminary findings from our survey of the online “Going Flat” community – patients who underwent mastectomy without breast mound reconstruction. I am happy to announce that our peer-reviewed manuscript* and accompanying editorial* have just been published in the Annals of Surgical Oncology.
I was inspired to research this topic as several studies have noted that patients who do not undergo reconstruction have poorer quality of life and satisfaction compared with those who have reconstruction. However, there are a growing number of support and advocacy groups dedicated to women who decline reconstruction, and we wanted to assess their experience. We partnered with patient advocates to ensure that we were asking appropriate and relevant questions. The results presented here are slightly different than the abstract – this is not uncommon as the abstract represents preliminary or “first review” findings. After doing a “deep dive” into the responses, our results are as follows:
Demographics and timing of going flat:
- 931 women completed the survey, mean age was 49 (range 25 – 85)
- Most patients were white (94%), had private insurance (71%), and were from the US (79%) although 22 countries were represented
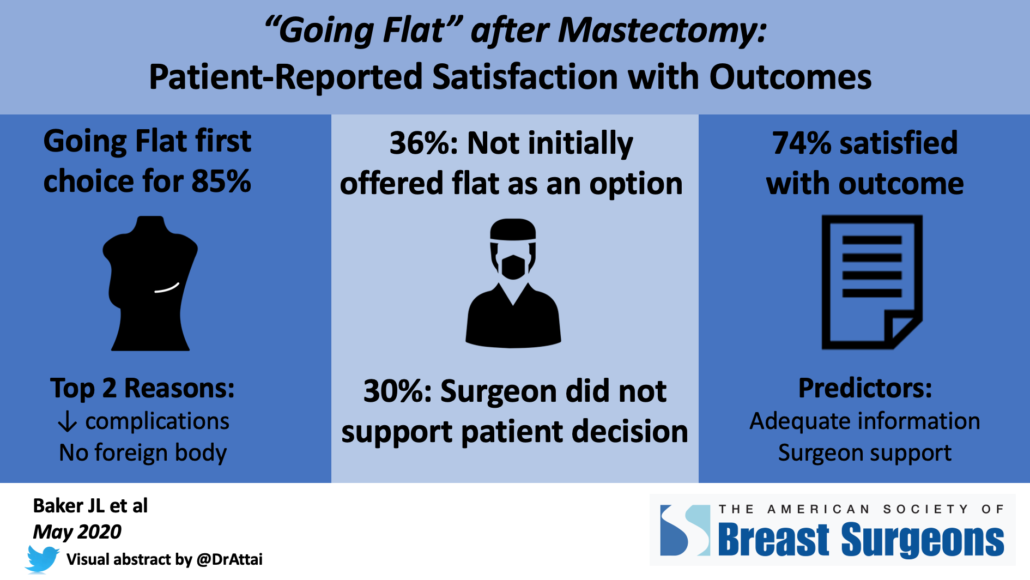
- 85% did not undergo breast mound reconstruction at the time of mastectomy
- 15% initially had reconstruction that was subsequently removed
Top reasons for going flat were desire to avoid a foreign body such as an implant, and perceived lower complication rate. 51% of patients stated that their breasts were not important for their body image.
Communication:
- 65% stated that they received adequate information about their surgical options
- 22% experienced “flat denial” – they were not initially offered the option to go flat, their surgeon was not supportive of their decision or tried to talk them out of the procedure, or extra skin was intentionally left in case the patient changed her mind.
Satisfaction with outcome:
- 74% agreed or strongly agreed that they were satisfied with their surgical outcome
- Strongest predictors of satisfaction were surgeon support of the patient’s decision to go flat and having adequate information about surgical options.
- Patients who reported that their surgeon had an exclusive breast surgery practice were less likely to be dissatisfied
- Factors most associated with flat denial include low level of surgeon support, high flat denial score, higher BMI (body mass index), and those undergoing unilateral (one side) versus bilateral (both sides) mastectomy
Our findings reveal a need for additional research into factors that impact patient satisfaction as well as for surgeon education on how to optimally support women who are not interested in breast mound reconstruction. In addition, surgeons should be trained in techniques to perform an aesthetic flat closure, or partner with their plastic and reconstructive surgical colleagues so that they can provide optimal results for their patients.
On behalf of my co-authors, we would like to thank all who shared and participated in the survey!

*If you are not able to access the full study and would like a copy, please email me: contact at drattai dot com