29 October 2015
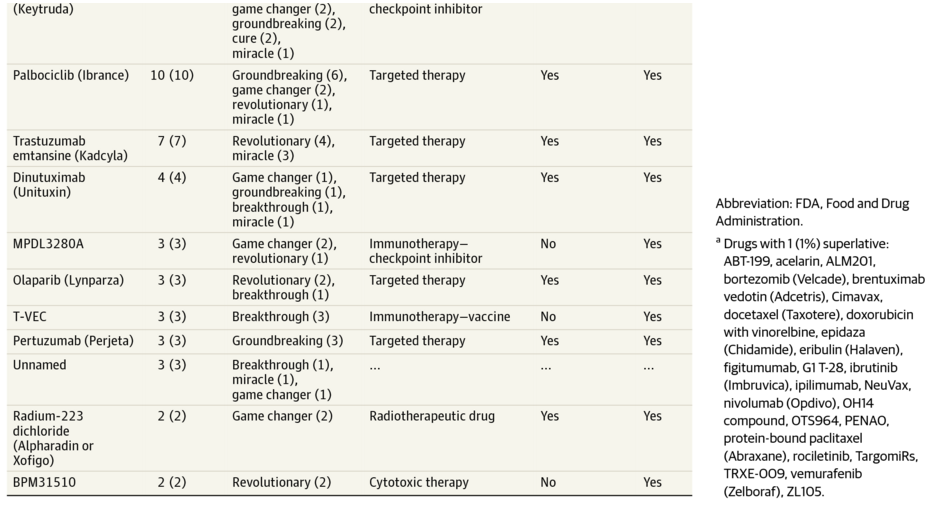
We’ve all seen the headlines noting a “blockbuster” or “groundbreaking” new drug for cancer treatment. It can be very difficult to sort out whether or not the hype is indicated. A study published in JAMA Oncology evaluated how news articles described new cancer medications. They evaluated the use of the superlative terms “breakthrough”, “game changer”, “miracle”, “cure”, “home run”, “revolutionary”, “transformative”, “life saver”, “groundbreaking”, and “marvel” in conjunction with “cancer drug”.
What they found was disturbing. 50% of the drugs described using the terms above were not FDA-approved. 14% had never been tested in humans – the research was performed in animal models and cell cultures. In the majority of cases, the superlative was used by the news article author, without attributing it to a physician or researcher. The study authors concluded that those using the terms may not necessarily have the expertise to properly evaluate research studies involving cancer medications.
What should the average patient do? It’s hard to ignore these stories – we are all on the alert for anything that could be considered a breakthrough in terms of cancer care. A good guide for reviewing news articles is posted at the nonprofit site Health News Review. Their 10-step process evaluates various components of the article, and allows even those with limited medical knowledge to critically review news articles. If it sounds too good to be true, it probably is.





Trackbacks & Pingbacks
[…] If It Sounds Too Good To Be True… […]
Comments are closed.