https://drattai.com/wp-content/uploads/2021/01/Screen-Shot-2021-01-12-at-3.59.34-PM.png
336
338
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2021-01-13 00:02:082021-01-13 00:02:10Tracking Breast Cancer Recurrence
https://drattai.com/wp-content/uploads/2021/01/Screen-Shot-2021-01-12-at-3.59.34-PM.png
336
338
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2021-01-13 00:02:082021-01-13 00:02:10Tracking Breast Cancer RecurrenceOpinion and Perspective
 https://drattai.com/wp-content/uploads/2021/01/Screen-Shot-2021-01-12-at-3.59.34-PM.png
336
338
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2021-01-13 00:02:082021-01-13 00:02:10Tracking Breast Cancer Recurrence
https://drattai.com/wp-content/uploads/2021/01/Screen-Shot-2021-01-12-at-3.59.34-PM.png
336
338
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2021-01-13 00:02:082021-01-13 00:02:10Tracking Breast Cancer Recurrence https://drattai.com/wp-content/uploads/2021/01/Screen-Shot-2021-01-04-at-9.59.06-AM.png
1122
2000
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2021-01-04 17:56:542021-01-27 16:26:06The “Going Flat” Patient Experience
https://drattai.com/wp-content/uploads/2021/01/Screen-Shot-2021-01-04-at-9.59.06-AM.png
1122
2000
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2021-01-04 17:56:542021-01-27 16:26:06The “Going Flat” Patient Experience https://drattai.com/wp-content/uploads/2020/06/Screen-Shot-2020-06-13-at-3.09.29-PM.png
1064
1208
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2020-12-29 18:52:002021-01-08 18:57:58Second Primary Cancers
https://drattai.com/wp-content/uploads/2020/06/Screen-Shot-2020-06-13-at-3.09.29-PM.png
1064
1208
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2020-12-29 18:52:002021-01-08 18:57:58Second Primary Cancers https://drattai.com/wp-content/uploads/2018/12/24158851_s.jpg
692
692
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2020-12-28 00:34:002021-01-07 00:41:25Pregnancy after Breast Cancer
https://drattai.com/wp-content/uploads/2018/12/24158851_s.jpg
692
692
drdeannaattai
https://drattai.com/wp-content/uploads/2019/06/logo.png
drdeannaattai2020-12-28 00:34:002021-01-07 00:41:25Pregnancy after Breast CancerBreast Health Resources
Media
Breast Cancer Treatment
- October 2021 – Yahoo Life – These Breast Cancer Survivors are Happier Than Ever Post-Mastectomy
- October 2021 – Cure Today – Some Women with Breast Cancer Report “Flat Denial”
- June 2021 – Cancer Today – Going Flat
- February 2021 – BreastCancer.org Podcast – Talking to Your Doctor about Going Flat
- September 2020 – Cancer Therapy Advisor – How Breast Cancer Patients Really Feel About Endocrine Therapy
Additional Information
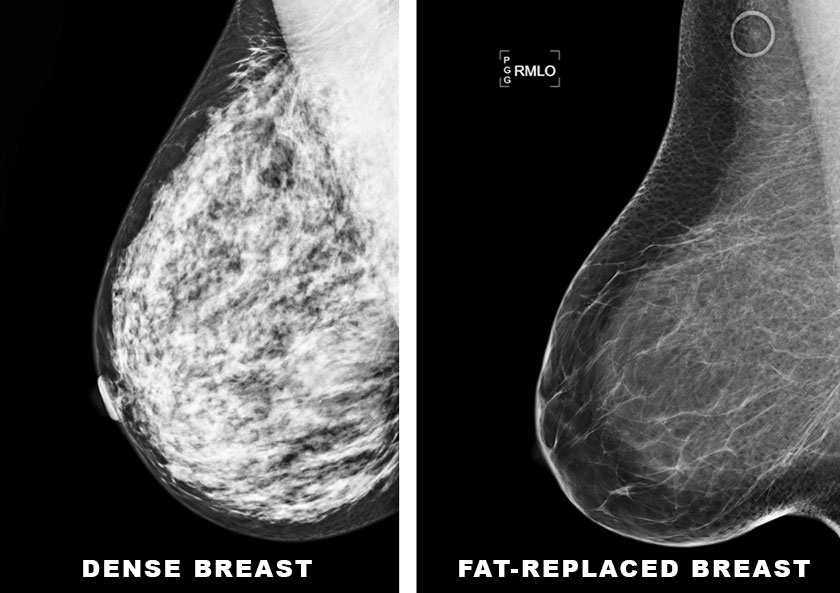
Breast Cancer Detection and Risk
Social Media
- October 2020 – Medscape – #BCSM: Invaluable Tool for the Breast Cancer Community
- October 2020 – UCLA Newsroom – How a Twitter Hashtag Provides Insights for Doctors and Support for for People with Breast Cancer
- August 2020 – Cancer Therapy Advisor – The Twitter Effect on How Oncologist Conduct and Share Research
- April 2019 – Cancer Therapy Advisor – Navigating Cancer Communication on Twitter
- February 2019 – Cancer Today – Considering the Term “Cancer Survivor”
- June 2018 – MedPage Today – #SocialMediaPrimer: Do’s and Don’ts for Cancer Docs
- March 2018 – Health News Review podcast – Doctors Who Blog
Additional Information
Contact
Dr. Deanna J. Attai
UCLA Health Burbank Breast Care
191 S. Buena Vista Street, Suite 415
Burbank, CA 91505
Phone: 818-333-2555
Fax: 818-333-2559
Email: [email protected]
DISCLAIMER:
This website does not provide medical advice and should not be used to diagnose or treat any medical condition. The opinions expressed on this website are those of Dr. Attai and do not reflect the views of the University of California Los Angeles or the David Geffen School of Medicine. Dr. Attai does not consult for or have financial relationships with any websites, companies or products, including those that are referenced on this website.