15 March 2020
Ductal carcinoma in-situ, also known as DCIS or stage 0 breast cancer, is traditionally treated with surgical excision (lumpectomy or mastectomy). Treatment may also include radiation therapy, as well as endocrine therapy (tamoxifen or an aromatase inhibitor) if the disease is estrogen receptor positive (ER+).
For invasive cancer, we sometimes take a neoadjuvant approach, treating with chemotherapy or endocrine therapy prior to surgery. This has the advantage of confirming that the tumor will actually respond to treatment. In addition, in cases where the tumor does not completely resolve with treatment (based on pathology assessment of the tissue that is removed), additional chemotherapy or targeted treatments may be recommended.
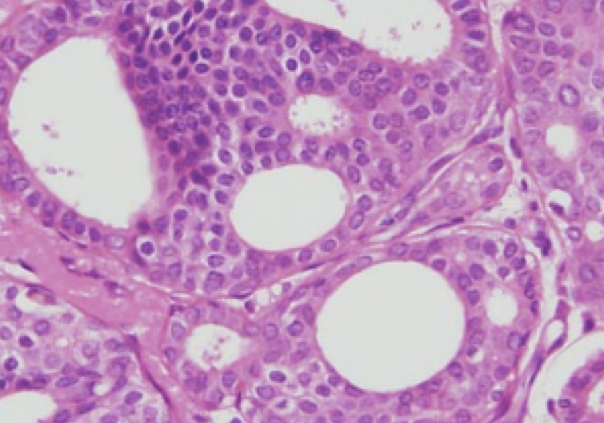
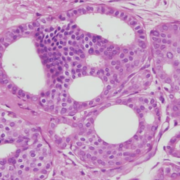
We have not traditionally used a neoadjuvant approach for DCIS. While invasive cancers may shrink in response to treatment, it is unclear if that reliably happens with DCIS. A study recently published used letrozole (Femara – an aromatase inhibitor) in patients with ER+ DCIS. Patients were treated for 6 months, had MRIs at baseline, 3 and 6 months, and then underwent surgery. The size of abnormality on MRI (which often, but not always correlates with the amount of disease) was measured, and ER, PR (progesterone receptor) and Ki67 (a measure of cellular proliferative activity) was assessed on the pre-treatment needle biopsy and on the surgical specimen. 79 patients were enrolled, 70 completed 6 months of letrozole, and MRI data for all 3 time points was available for 67 patients.
The study found that:
- Median volume of disease as measured by MRI declined by 0.8cm
- ER and PR H-scores decreased by a median of 15 and 85 points, respectively
- Ki67 decreased by a median of 6.3%
Of the 59 patients who underwent surgery, findings included:
- Persistence of residual disease in 50 patients (85%)
- Invasive cancer in 6 patients (10%)
- No residual DCIS and no invasive cancer found in 9 patients (15%)
As mentioned above, the finding of residual disease is not unexpected. DCIS does not often resolve after neoadjuvant therapy, and endocrine therapy works very slowly. In addition, several patients were found to have invasive cancer – the authors suspect that most likely this was not picked up on the initial biopsy as we know the “upstaging” rate for DCIS at surgery can approach 25%. However, despite these limitations, the finding of decreased volume of disease by MRI and changes in biomarkers (ER, PR and Ki67) indicate treatment response and suggest that extended neoadjuvant endocrine therapy may eventually play a role in the treatment of ER+ DCIS and may possible replace surgery in selected patients. This was a relatively small Phase II trial, so more study certainly is needed.
*If you are not able to access the full study and would like a copy, please email me: contact at drattai dot com
- Additional Information:
- ASCO Post
- Journal of Clinical Oncology editorial