19 January 2015
This past weekend, I gave a talk at the Southern California Chapter of the American College of Surgeons Annual Meeting – the title of the talk was Increasing Mastectomy Rates – Science vs. Personal Choice.
There is a tremendous amount of literature documenting the increasing mastectomy rates. The talk focused on women with early stage breast cancer at average risk for developing a recurrence or new cancer – women without a BRCA gene mutation. As has been my practice for several years when giving a talk which includes the patient experience, I asked you for input, and received a lot of information. The following is a summary of the talk, including your perspective.
The use of mastectomy for breast cancer has been documented as early as the 1500s, despite the fact that general anesthesia did not come into use until the 1840s. Sir William Halsted described the radical mastectomy, which involved removal of the breast, all of the overlying skin, the pectoral (chest) muscle, and a significant number of lymph nodes. It was a very aggressive surgery but at the time, many women at the time presented with advanced disease – cancers that grew through the skin or chest muscle. The Halsted theory was that if the breast and lymph nodes could be removed with an extensive “en bloc” surgery, the cancer had a lower likelihood of spreading. However, despite this aggressive approach, the dismal survival rates from breast cancer did not improve.
Halsted died in 1922, but the radical mastectomy remained the surgical procedure of choice until the 1960-70s. The landmark NSABP B04 trial, led by Dr. Bernard Fisher, demonstrated that regardless of surgical decision (radical mastectomy vs. total mastectomy – no removal of the muscle) the survival rates were equivalent, and these results have held up for 25 years(1). The Fisher theory was that cancer may be metastatic from the beginning, and that a more extensive surgical procedure would not be expected to improve survival rates. The NSABP B06 trial demonstrated equivalent survival rates whether women underwent mastectomy, lumpectomy / radiation, or lumpectomy alone. However, if radiation therapy was not performed, the risk of local recurrence (cancer returning in the breast) was 39.2%, compared to 14.3% with radiation(2). This is the basis for our current recommendation of lumpectomy followed by radiation therapy for early stage breast cancer. A 1990 NIH consensus panel stated that “breast conservation treatment is an appropriate method of primary therapy for the majority of women with early-stage breast cancer and is preferable because it provides survival rates equivalent to those of mastectomy while preserving the breast’’(3).
This was seen as a major scientific advance, and one that was embraced by patients – no longer did women need to have a breast removed for early stage disease, and from the early to mid 1990s, lumpectomy rates started increasing while mastectomy rates decreased.
The Women’s Health and Cancer Rights act of 1998 stated that if an insurance company covered the procedure of mastectomy, they were required to cover reconstructive surgery, including procedures performed on the other breast to produce a symmetrical appearance, as well as prosthetics for lymphedema. This set the stage for immediate reconstruction, which prior to this time was generally not performed (or recommended) on a regular basis.
We think of the “Celebrity Effect” when we hear Angelina Jolie, Amy Robach, and others discuss their decisions. But in 1987, Nancy Regan underwent a mastectomy for breast cancer, and she received a significant amount of criticism for her decision, both from the medical community as well as from advocacy groups. Women who underwent breast cancer surgery from the end of 1987 to early 1988 were 25% less likely to undergo lumpectomy compared to earlier in 1987, prior to her diagnosis(4). Lumpectomy rates subsequently increased, but she later wrote “This is a very personal decision, one that each woman must make for herself. This was my choice, and I don’t believe I should have been criticized for it”(5).
Around 2004, it was noted that mastectomy rates started rising(6). This trend was seen nationally as well as in many individual institutions.
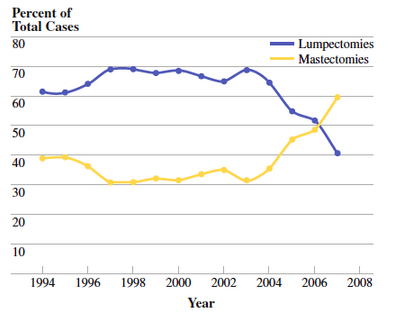
In addition to an increase in mastectomies being performed for early stage breast cancer, an increase has been seen in contralateral prophylactic mastectomy – removal of the other, non-cancerous breast. Many studies have been published confirming this trend, and also evaluating factors associated with an increased mastectomy rate(7,8,9). They include:
– Young age, Caucasian race
– Higher economic status, better insurance, availability of reconstruction
– Family history of breast cancer or genetic testing – even if the genetic testing was negative
– Undergoing an MRI, even if the MRI was normal
Patient factors have also been evaluated. Many studies have cited that women make their decisions out of fear. Interestingly, there seems to be some intellectual disconnect – women report that they understand there is no improvement in survival, yet state that they made their decision “to live longer”. The physician has been identified as a very important source of information, yet only 1/3 of women stated that a desire to follow their physician’s recommendation was important in making their decision. Many women over-estimate their risk of developing a new breast cancer – some reporting they think their risk is as high as 50%. Other studies have reported that women make their decisions to gain a sense of control over cancer, but that many have an exaggerated sense of control, stating that they are making the decision “so I don’t have to go through this again”, while admitting that they are aware that mastectomy does not reduce the rate of metastatic disease. The impact of a family member / friend experience was also noted to be very important. All of us make decisions in our daily lives based on personal experience rather than hard facts – a woman facing a decision about breast cancer surgery is no different. Finally, many woman remain very satisfied with their decision even 20 years after the surgery. However, it is important to note that 10-30% report issues related to self-esteem, body image, sexuality, emotional stability, and overall quality of life (10, 11, 12).
Some facts(10, 13, 14, 15):
– For women at average risk of breast cancer (BRCA negative) the rate of developing a new breast cancer is approximately 0.5 – 0.75% per year. This can be reduced if the women undergoes chemotherapy and/or endocrine therapy
– Mastectomy for early stage breast cancer or contralateral prophylactic mastectomy does not reduce the likelihood that breast cancer will metastasize (spread to other areas of the body)
– The complication rate increases with more surgery – bilateral mastectomy is associated with 30-40% risk of complications including infection, fluid accumulation, and re-operation.
So what did you, the #BCSM Community have to say? Out of those who responded:
– “I knew survival rates were the same” – most patients well informed, had surgeons who presented all sides, supported their decision
– 40% said decisions influenced by family/friend experience
– 15% had lumpectomy initially, then opted for bilateral mastectomy after anxiety of repeat imaging and biopsies.
– 6 patients: lumpectomy and radiation: significant problems with wound healing, fibrosis and later underwent a mastectomy
– 5 patients subsequently developed metastatic disease; no regrets on their decision
– 3 patients required multiple surgeries due to revisions, infection, lost implant – no regrets
– 2 patients felt pushed into their decision, one by family members and another by their physician – both regretted their decision
There are a lot of comments on the original blog post; here are a few I received by email:
– “I wish doctors, researchers and the media understood (some do) – there are many valid reasons for choosing a mastectomy, even with the state-of-science today”
– “The focus is on ‘simple’ surgery – the potential toxicity of radiation therapy is grossly minimized. While serious and long-term side effects of radiation therapy may be rare, they do occur. It is ironic now that patients have a choice in treatment selection, there is so much hand-wringing by the medical establishment in the choices that many women make”
– “We are diligent. We are thoughtful. We have good reasons for choosing the “big surgery”. Our doctors explain the risk factors, we process the information, we understand the full ramifications of our choice, and are still confident that this is the right choice for our set of circumstances.
– It may not fit the medically necessary criteria, but it may fit with the emotionally necessary criteria. I hear your evidence based science and I’ll raise you five intangibles…”
So what is the answer? Clearly physicians have a responsibility to educate our patients not only on the lack of overall survival benefit, on the complication rates. Physicians also need to do a better job of assessing and explaining a patient’s risk of developing a recurrence or a new breast cancer. And patients should be encouraged to take their time, obtain opinions, and carefully consider all options prior to making a decision. But rather than irrational fear, what many of see in our practice is “Reasonable Fear”. Patient’s biases and personal experiences need to be acknowledged. Some bias, but not all, can be overcome with education. But until science advances to allow us to truly predict who will and will not develop a recurrence or a new breast cancer, personal choice should remain an option.
References:
1. Fisher B et al. 25 Year follow up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. NEJM 2002;347 (8)
2. Fisher B et al. 20 Year follow up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer NEJM 2002;347 (16)
3. NIH Consensus Conference: Treatment of Early Stage Breast Cancer. JAMA 1991 265
4. Nattinger AB, et al Effect of Nancy Regan’s mastectomy on choice of surgery for breast cancer by US women. JAMA 1998 (279) 10 762-766
5. Olson, J: “Bathsheba’s Breast: Women, Cancer and History” The Johns Hopkins University Press 2002
6. McGuire KP, et al Are mastectomies on the rise? A 13 year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 2009 16:2682-2690
7. Mahmood U, et al Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol 2013 20:1436-1443
8. Yao K, et al Trends in contralateral prophylactic mastectomy for unilateral cancer: A report from the national cancer database 1998-2007. Ann Surg Oncol 2010(17) 2554-2562
9. King TA, et al Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol 29:2158-2164 97-200
10. Rosenberg SM, et al Perceptions, knowledge and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer Ann Intern Med 2013;159:373-381
11. Covelli AM, et al ‘Taking control of cancer’: Understanding women’s choice for mastectomy. Ann Surg Oncol DOI 10.1245/s10434-014-4033-7
12. Frost MH, et al Contralateral prophylactic mastectomy: Long term consistency of satisfaction and adverse effects and the significance of informed decision making, quality of life, and personality traits. Ann Surg Oncol 2011 18:3110-3116
13. Fayanju O et al Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg 2014; 260:1000-1010
14. Roberts A et al Cost effectiveness of contralateral prophylactic mastectomy for prevention of contralateral prophylactic mastectomy. Ann Surg Oncol 2014 21:2209-2217
15. Miller ME et al Operative risks associated with contralateral prophylactic mastectomy: A single institution experience. Ann Surg Oncol 2013 204113-4120.










 Dr. Oliver Bogler is a cancer researcher, male breast cancer patient, and male breast cancer advocate. His blog can be found at
Dr. Oliver Bogler is a cancer researcher, male breast cancer patient, and male breast cancer advocate. His blog can be found at 